FOR PARENTS
Osteopathic Plagiocephaly Treatment for Infants & Neonates (OPT-IN) Study
This is a clinical trial for infants 4 months of age and younger who are diagnosed with Deformational or Positional Plagiocephaly (flat head syndrome). The study offers alternative treatments to helmet therapy.
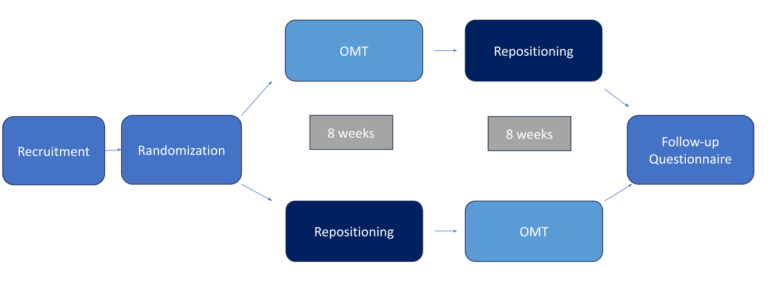
The study will last approximately 1 year. However, the majority of your participation will occur during the 16-week period after your child begins. Your infant will be screened and evaluated to determine if they can participate. If eligible, randomly assigned to one of two groups:
- The Osteopathic Manipulative Treatment (OMT) group, or
- The Standard of Care Repositioning group

How OPT-IN Works
Step 1: Determine if your child is eligible
Complete our pre-screening survey (request it below).

Step 2: Consent to Study participation
Both parents/guardians for the child need to consent to study participation.

Step 3: participate in the study
Your child will participate in both of our study groups (each one lasts for 8 weeks). When your child is in the OMT group you will visit us weekly for treatments and measurements. When your child is in the Standard of Care repositioning group, you will do exercises at home using handouts as your guide and visit us twice for measurements. You will be providing us information on your child through different questionnaires while they participate.

Step 4: provide follow-up feedback
When your child is 12 months old we will send a follow-up survey for you to complete about their health and development.
Join the Study
Request our pre-screening survey below or contact us directly at OPT-IN@the-promise.org or 619-693-6303. Se habla Español.
Your Child's Eligibility
Your child is eligible if…
- They are 4-months of age or younger (between birth – 4 months) at the time of enrollment
- They were diagnosed with deformational or positional plagiocephaly (flat head syndrome)
by a healthcare provider
Your child is NOT eligible if…
- They are 5 months or older
- They were born preterm (before 37 weeks)
- They were diagnosed with any genetic syndromes
- They were diagnosed with craniofacial defects such as craniosynostosis
- They were diagnosed with hypertonia or hypotonia
- They sustained head trauma or neurological illnesses
- They have had prior helmet therapy, physical therapy, or Osteopathic Manipulative Treatment (OMT)
If you still have questions about whether or not your child might be able to participate, please see the contact information below and our team is happy to help!
Our Research Team

Kimberly Wolf, DO, FAAP, FACOP, FAAPO
Dr. Wolf is a graduate of Seton Hall University, South Orange, NJ and Western University of Health Sciences, College of Osteopathic Medicine of the Pacific (COMP), Pomona, CA. She is board certified in Pediatrics. Dr. Wolf was a dually accredited Pediatric Resident at Nationwide Children’s Hospital and Doctor’s Hospital, Columbus, OH. Dr. Wolf is a Fellow of the American Academy of Pediatrics, the American College of Osteopathic Pediatricians and the American Academy of Pediatric Osteopathy. She is currently an Associate Professor of OMM and Director of Pediatric OMM at Touro University California College of Osteopathic Medicine and Research Director for the Osteopathic Center for Children. Her memberships include: AOA, AAO, ACOP, AAP, AAPO, and OCA.

Hollis King, DO, PhD, FAAO, FCA, FAAPO
Dr. King is a graduate of Texas College of Osteopathic Medicine, Fort Worth, TX. His postdoctoral training was at Dallas Memorial Hospital, Dallas, TX. His internship was at the VA Hospital & Univ. of Kentucky Medical School, Lexington, KY. His Ph.D. in Clinical Psychology is from Trinity University, San Antonio, TX. Dr. King is board certified in Family Medicine/OMT from the American Osteopathic Board of Family Physicians and certified with Special Proficiency in Osteopathic Manipulative Medicine from the American Osteopathic Board of Neuromusculoskeletal Medicine. He is a Founding Diplomate of the American Board of Holistic and Integrative Medicine. He is a Fellow of and Past-President of the American Academy of Osteopathy. He is a Fellow of the Osteopathic Cranial Academy. Recently Dr. King was made a Fellow of the American Academy of Pediatrics. Dr. King recently retired as Clinical Professor of Family Medicine at the University of California San Diego School of Medicine. He has a private practice at Osteopathic Center San Diego. Dr. King is Research Director at Osteopathy’s Promise to Children. His memberships include: AOA, AAO, OCA and AAPO.

Jessica Martone, PhD, MSW
Dr. Martone obtained her PhD and MSW from Loyola University Chicago and has extensive experience conducting research and evaluation. She was the Director for Research and Evaluation at The Mark USA and oversaw 60 concurrent projects in the areas of Science, Technology, Engineering, and Math. Jessica designed the research and evaluation methodology, conducted data collection and analysis and developed and disseminated findings. While at Loyola University Chicago Jessica worked for the Institute on Migration and International Social Work and contributed to several immigration focused research projects, including a global study on immigrant integration to identify best practices for social and economic integration. Jessica was an adjunct faculty member at Cal State Fullerton and taught the Research Capstone course and was an adjunct faculty member at Loyola University Chicago where she taught introduction to social work policy and practice courses. Dr. Martone is a subject matter expert approved by the OPC educational planning committee.

Mary Anne Morelli Haskell, DO, FACOP, FCA
Dr. Haskell is a graduate of the College of Osteopathic Medicine of the Pacific of Western University and completed a year of training in general medicine and then three years of pediatric residency at Loma Linda University Medical Center. She is board certified by the Osteopathic National Boards. She is boarded by the American Osteopathic Board of Pediatrics and the American Osteopathic Board of Neuromusculoskeletal Medicine. She holds a Certificate of Proficiency in Osteopathy in the Cranial Field from the Osteopathic Cranial Academy and is a Fellow of the Osteopathic Cranial Academy. The College d’Etudes Osteopathiques presented Dr. Haskell with the Viola Frymann award for excellence in osteopathic pediatrics. Her memberships include: AOA, AAO, ACOP, and OCA.

Julie Mai, DO
Dr. Mai is a graduate of the University of California, San Diego, CA and Touro University College of Osteopathic Medicine, Vallejo, CA. Dr. Mai completed a traditional internship at Chino Valley Hospital in Chino, CA. She holds a Certificate of Proficiency in Osteopathy in the Cranial Field from the Osteopathic Cranial Academy. She serves on the Boards of Osteopathy’s Promise to Children, Osteopathic Center for Children and the Osteopathic Cranial Academy. She served as the President of the Osteopathic Center for Children, and is currently President-Elect for the Osteopathic Cranial Academy. Her memberships are AOA, AAO, OCA, AAPO.
Frequently Asked Questions
Positional or deformational plagiocephaly (play-gee-o-SEF-uh-lee) is a flat area on the back or on the side of a baby’s head. This may happen from laying on their back to sleep, long or difficult labors, or sometimes how they were positioned when in the womb. It can be more commonly seen if the parent was pregnant with multiple children, the baby was premature, or if they have torticollis (tight muscle that limits neck motion).
We cannot guarantee any benefits to your child or others from taking part in this research. Possible benefits to your child may include: improved head shape, and improvement in degree of plagiocephaly (more symmetry in the head). We don’t know if there will be any other benefits besides the improved shape of your child’s head, but some infants who receive OMT also have improvements in other conditions such as colic, reflux (spitting up), feeding problems (e.g., latch difficulties), sleepiness, fussiness, and torticollis (restricted neck motion or tight neck muscles). This new knowledge will also benefit future patients and medical investigators.
The OMT techniques used in this protocol are gentle and well-tolerated on babies. Patients rarely experience side effects. If they do experience side effects, they are minor side effects. However, as with any medical procedure, OMT carries some risks. The most important risks or discomforts that you may expect from taking part in this research include:
Likely:
Minimal discomfort/fussiness during treatment for your baby
Sleepiness following treatment (many take a long nap)
Less common:
Fussiness or some irritability following treatment
Soreness following treatment
Patients will be assessed for all of these at each visit through observation of vital signs, physical examination findings, feeding behaviors, sleep behaviors, and irritability/fussiness. There may be unknown side effects but we do not anticipate these as this is a treatment that has been used for over 100 years. It is important to call the researcher or your regular physician when you think your infant is having problems, even if they are not included on the above list.
Participants can receive up to $550 compensation for study completion paid in the form of Target GiftCards. Your compensation will be broken down as follows:
- You will receive a $40 Target GiftCard for each treatment visit for a total of 8 OMT visits (up to $320).
- You will receive an additional $150 Target GiftCard once all 8 OMT visits are completed.
- You will receive a $30 Target GiftCard for each measurement visit (2 total).
- You will receive a $20 Target GiftCard after completing the 12-month survey.
DISCLAIMER:
Target and the Bullseye Design are registered trademarks of Target Brands, Inc. Terms and conditions are applied to gift cards. Target is not a participating partner in or sponsor of this offer.
If you take part in this research, you will be responsible for:
- Completing an intake/health history questionnaire.
- When in the OMT group:
- Attending 8 OMT sessions (~1 session/week) at an outpatient clinic. These will be ~30-45 minutes long.
- At each of these visits, your child’s head will be measured with a device called SoftSpot™ a technology where a soft cap is placed on the baby’s head and then a picture is taken from the top of the baby’s head. You will not be able to identify your child from these pictures and they will be protected in a secure database for the study.
- When in the Standard of Care Repositioning group:
- Providing standard-of-care repositioning for 8 weeks. This consists of simple exercises at home such as increasing tummy time and actively making your child look both ways (see Repositioning Education handout below).
- Attending two clinic visits for measurements: one prior to beginning repositioning and the second after 8 weeks of repositioning. These will be ~15 minutes long. These measurements will also be taken with SoftSpot™.
- Providing feedback during the treatment on any physical, behavioral, or medical conditions that may result after OMT sessions and also using a short weekly interval (follow-up) questionnaire.
- Completing an electronic survey when your child is 12 months old that will ask about their health and their development.
- Refraining from obtaining helmet therapy or other corrective cranial devices/orthotics for plagiocephaly, or engaging in physical therapy or other treatments for plagiocephaly, or other OMT throughout the duration of the 16 weeks. They may be utilized after the 16 weeks of OMT + repositioning if that is recommended by the child’s doctor and/or your family desires.
Status: Accepting new patients
Start Date: November 2023
Completion Date: December 2024 (estimated)
Sponsor: Osteopathy’s Promise to Children
Participants: Aiming to enroll 122 study participants
ClinicalTrials.gov: NCT05848895
Last Updated: February 2024
Join the Study
Request our pre-screening survey below or contact us directly at OPT-IN@the-promise.org or 619-693-6303. Se habla Español.
